biopix-t introducing
Diagnostics
ecosystem
With a massive upgrade in diagnostics and affordable, customizable and scalable devices, Biopix-T’s Ecosystem is a remarkable innovation.
Chemistry
Hardware
Data
Software
Ecosystem Benefits
Instant test design, with no limitations
Lab grade performance
Cost efficient solutions
PixL
The world’s smallest molecular diagnostic device for accurate and affordable home testing.
Outperforming lateral flow tests and poised to become the fast-test new standard of care.
Trustworthy
User Friendly
Innovative
Affordable to all
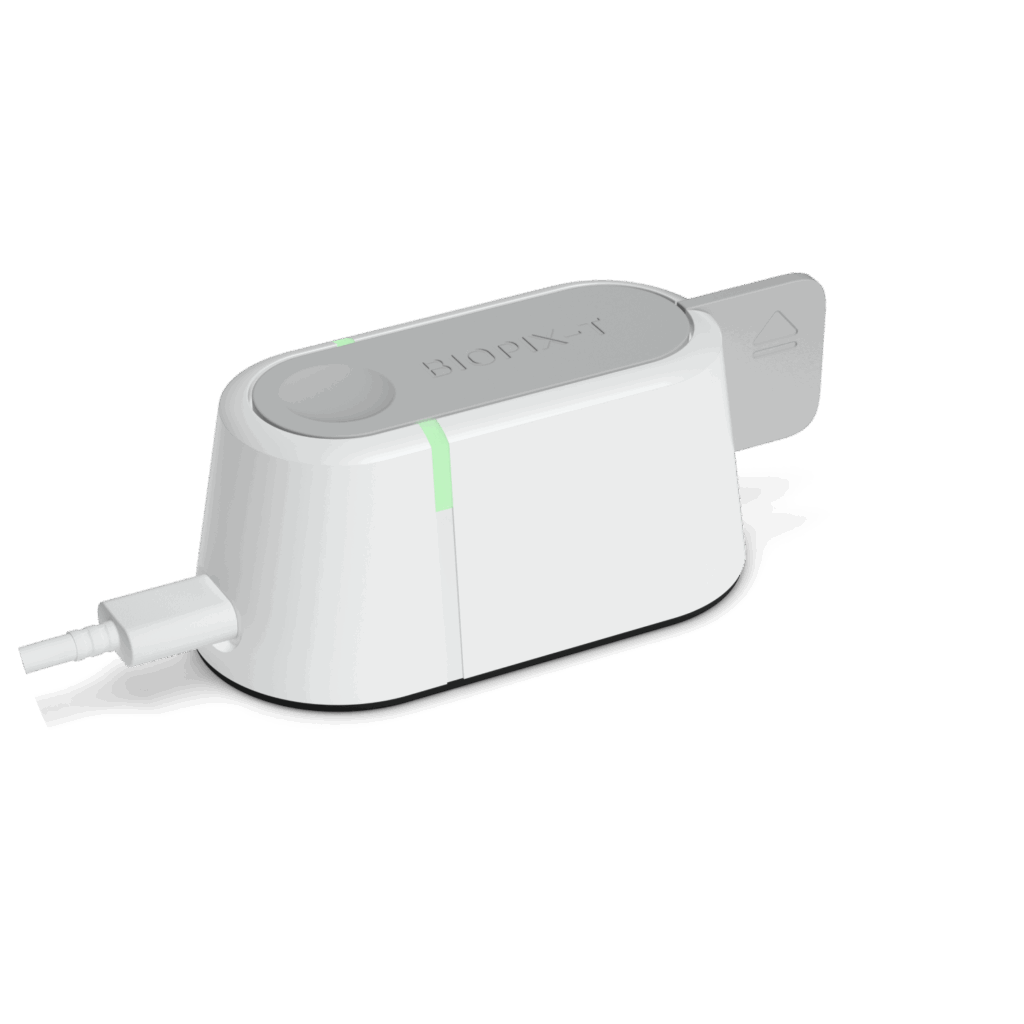
Pebble
for decentralized testing

Sample-to-answer in 15 to 30 min
Quantitative
User-friendly
Lightweight
Low power consumption
Smartphone controlled
Connected
Affordable to all
Pebble
Technology Description
Pebble is a compact device for performing real-time colorimetric LAMP (qcLAMP). Pebble was created using three-dimensional (3D) additive manufacturing technology and operates via an Android application.
The device can be used to extract rapidly quantitative information at a wide dynamic range of RNA or DNA template. Detection can occur from purified nucleic acids or directly from crude samples (swabs, saliva and biopsy tissue).
Our vision
Our mission

